This fact box is is one of three fact boxes designed to help you weigh the benefits and side effects of the MMR combination vaccination against measles, mumps, and rubella in young children. The focus of this fact box is on measles. The information and figures do not offer a definitive assessment. They are based on the best current scientific evidence available.
The fact box was developed by the Harding Center for Risk Literacy.
This fact box is is one of three fact boxes designed to help you weigh the benefits and side effects of the MMR combination vaccination against measles, mumps, and rubella in young children. The focus of this fact box is on measles. The information and figures do not offer a definitive assessment. They are based on the best current scientific evidence available.
The fact box was developed by the Harding Center for Risk Literacy.
Measles is a highly contagious infectious disease for both children and adults, caused by the measles virus. Even brief contact with an infected person can pass the virus on through the air and droplets transmitted when coughing, talking, and sneezing, or through contaminated surfaces and objects (e.g. door handles, drinking bottles) [1, 2].
In the first phase of the disease, symptoms such as fever, cough, cold, conjunctivitis, and white spots on the inside of the cheeks are common. In the second phase of the disease, a splotchy, bright red skin rash typical of measles appears. Fever can rise sharply and lead to a febrile convulsion. The rash starts on the face and behind the ears and then spreads to the rest of the body [1,2].
Although measles usually has no serious consequences, it is not harmless. Measles can weaken the body's immune system for months to years. Other pathogens are then more difficult to fight off. That can lead to infections of the middle ear or larynx or to pneumonia. About 10 out of 10,000 children and adolescents with measles develop a brain infection, from which 1 to 2 die and 2 to 3 experience severe secondary damage such as intellectual disabilities [1, 2].
Subacute sclerosing panencephalitis (SSPE) develops in about 1 to 5 out of 10,000 cases of measles. This very rare and severe inflammation of the brain can occur years after a person has had measles and leads to a progressive disease of the nervous system that is usually fatal [1].
Measles can also occur in a rare and mild form, with few symptoms and a barely visible skin rash. This form (mitigated measles) is nevertheless contagious and tends to occur in newborns and in people who have only been vaccinated once and do not have full protection against the measles virus [1].
In Germany, it is mainly unvaccinated 0- to 5-year-old children, adolescents and younger adults who catch measles [2].
The measles-containing vaccine is given twice to infants as part of a triple combination vaccination also for mumps and rubella (MMR) or as part of a quadruple combination vaccination also for mumps, rubella, and chickenpox (MMRV). After a double vaccination, a lifelong immunity to measles is generally assumed so that, according to the current state of science, vaccination protection does not need to be refreshed later. In most cases, full vaccination protection exists three to four weeks after the second vaccine dose [2].
The Standing Committee on Vaccination (STIKO) of the Robert Koch Institute (RKI) recommends administering the first MMR(V) vaccine dose as part of basic immunization. This takes place at the age of 11-14 months. The second MMR(V) vaccine dose can be given at the earliest four weeks after the first dose and the vaccination should thus be completed by the age of 15-23 months. The second vaccine dose is considered a precautionary measure in case sufficient immunity was not developed after the first vaccination [2].
Furthermore, all adults born after 1970 who were not vaccinated in childhood, who were vaccinated only once, or whose vaccination status is unclear may consider a single MMR(V) combination vaccination [2].
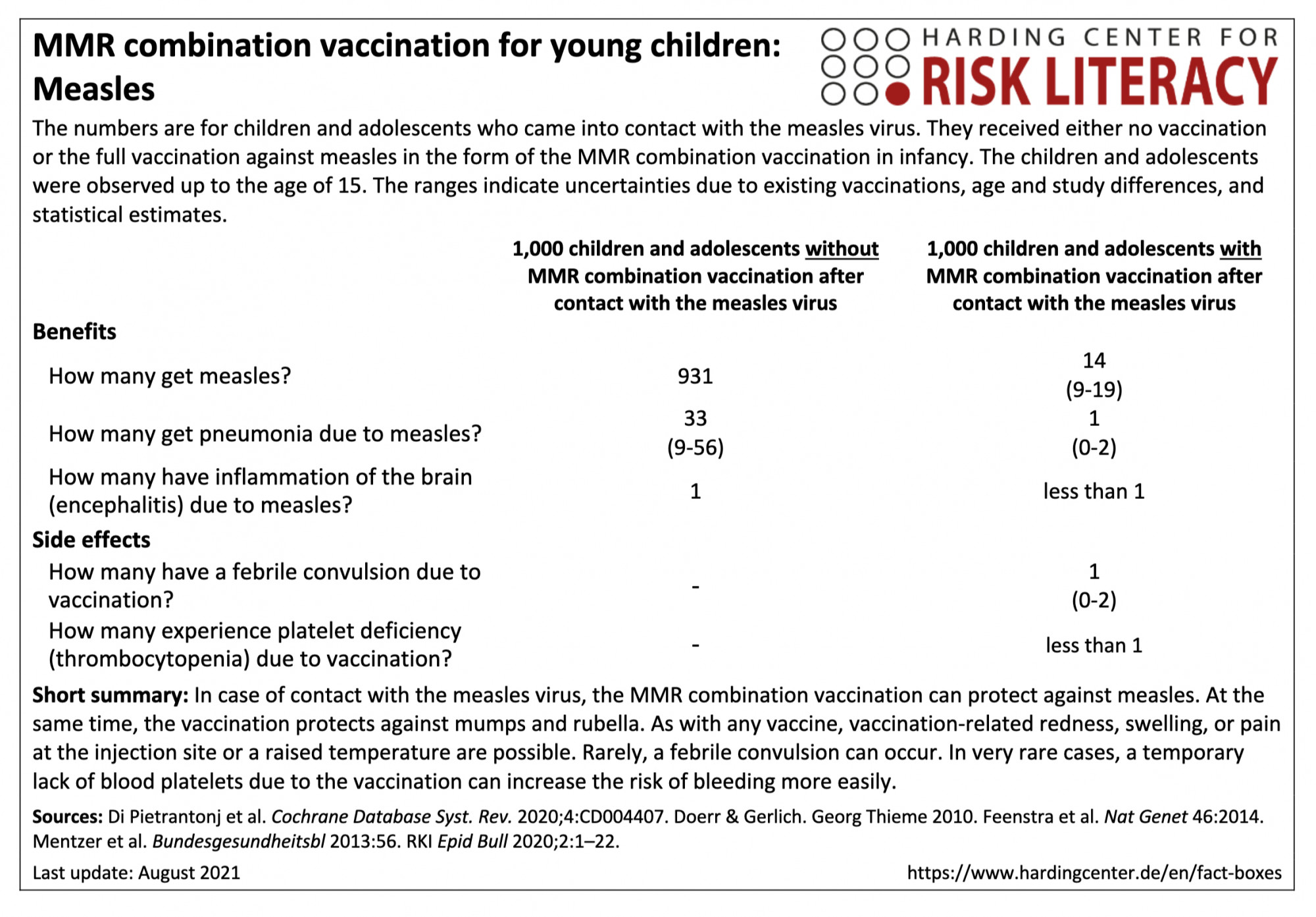
In the fact box, the benefits of the MMR combination vaccination and of non-vaccination are compared with each other as a model for children and adolescents up to the age of 15 who come into contact with the measles virus. In addition, the side effects of the MMR combination vaccination are compared with those of non-vaccination.
The table may be read as follows:
If unvaccinated children and adolescents came into contact with the measles virus by the age of 15 years, about 931 per 1,000 would likely get measles. In contrast, an average of 14 (9 to 19) per 1,000 of those vaccinated with an MMR combination vaccine would get measles after contact with the virus [2, 3]. This means that on average 917 out of every 1,000 children and adolescents can be prevented from getting measles by the MMR combination vaccination.
The MMR combination vaccination can cause febrile seizures in about 1 (0 to 2) out of every 1,000 infants [4].
The figures on the frequency of illness after contact with the measles virus are based on model calculations with collected data from clinical practice. This includes medical information and textbook data on contact indices (proportion of infected persons among those who have contact with the virus) and manifestation indices (proportion of symptomatically ill persons among those infected). The figures based on clinical practice do not necessarily correspond to the health status of today's population and all vaccines currently offered in Germany [1-3].
The figures on side effects are from various studies with different study designs and populations, with a total of about 2,152,000 participants [4,5].
| 0-2 Monate | 3 Monate - 5 Jahren | 6 Jahren - 14 Jahren | Ab 15 Jahren | |
| Frauen | - | X | (X) | - |
| Männer | - | X | (X) | - |
Erklärung der Symbole: X = für diese Personen gelten die Zahlen in der Faktenbox; (X) = auf diese Personen lassen sich die Zahlen unter Vorbehalt anwenden (in solchen Fällen ist eine Rücksprache mit ärztlichem Personal empfehlenswert); - = für diese Personen gelten die Zahlen nicht; ? = es ist unbekannt, ob die Zahlen für diese Personen gelten
Because the MMR(V) combination vaccination is a live vaccination with attenuated mumps, measles, and rubella viruses (and chickenpox), mild, non-transmissible "vaccine measles" may occur one to four weeks after vaccination in about 20 to 50 infants out of every 1,000 vaccinated. This is accompanied by fever and a mild measles-like rash. Mild swelling of the parotid glands (swelling of the cheeks) is also occasionally possible. Joint pain has been reported in adolescents and adults (very rarely in children). Rarely, mild swelling of the testicles has been observed. Such reactions to the MMR(V) combination vaccination are usually temporary and subside on their own without any further consequences [2].
The likelihood of contact with the measles virus depends on many factors. These include, for example, the number of vaccinated people in the population and the possibilities for the measles virus to spread. Contacts with the pathogen become less frequent if a majority of the population is vaccinated, which prevents the virus from spreading (herd immunity) [1].
There is no evidence for a connection between the MMR(V) combination vaccination and the occurrence of inflammatory bowel diseases, such as chronic inflammation of the gastrointestinal tract (Crohn's disease) or of the colon (ulcerative colitis). There is also no link between the occurrence of autism (profound developmental disorder) or progressive, usually fatal brain damage (subacute sclerosing panencephalitis (SSPE)) and the vaccination [1-2, 5-8].
Furthermore, there is no evidence for a connection between the MMR(V) combination vaccination and cognitive developmental delays, the development of type 1 diabetes, asthma, chronic inflammatory skin diseases (dermatitis/eczema), hay fever, blood cancer (leukaemia), the chronic inflammatory neurological autoimmune disease multiple sclerosis, gait disorders, and bacterial or viral infections [5].
The evidence base was determined by the authors of the included review and the creators of the fact box. The overall quality of the evidence is moderate.
The figures on the benefits of MMR(V) combination vaccination were extrapolated using contagion and manifestation indices. These have not been explored with randomized controlled trials. However, vaccine effectiveness has been confirmed across different study populations with different study designs. These demonstrate a reduction in disease symptoms in vaccinated versus non-vaccinated individuals.
The findings on seizures due to fever and platelet deficiency could be modified by further research (moderate evidence).
- August 2021 (update of the search, update of the evidence, update of the accompanying text)
- April 2016 (development)
Information for the fact boxes were obtained from the following sources:
[1] Institute for Quality and Efficiency in Health Care (IQWiG). (2020, 2 November). Measles. gesundheitsinformation.de. https://www.gesundheitsinformation.de/masern.html (11.08.2021).
[2] Robert Koch Institute (RKI). Communication of the Standing Commission on Vaccination at the Robert Koch Institute: Recommendation and scientific rationale for the alignment of occupationally indicated measles-mumps-rubella (MMR) and varicella vaccination. Epid Bull 2020;2:1-22. DOI: 10.25646/6447.3.
[3] Doerr, H. W. & Gerlich, W. H. (2010). Medical virology. Thieme. DOI: 10.1055/b-001-2163.
[4] Feenstra, B., Pasternak, B., Geller, F. et al. Common variants associated with general and MMR vaccine-related febrile seizures. Nat Genet 46, 1274-1282 (2014). DOI: 10.1038/ng.3129.
[5] Di Pietrantonj, C., Rivetti, A., Marchione, P., Debalini, M. G., & Demicheli, V. (2020). Vaccines for measles, mumps, rubella, and varicella in children. Cochrane Database Syst Rev, (4). Art. No.: CD004407. DOI: 10.1002/14651858.CD004407.pub4.
[6] Federal Centre for Health Education (BZgA). (n.d.). Measles vaccination in children. infektionsschutz.de.
https://www.impfen-info.de/impfempfehlungen/fuer-kinder-0-12-jahre/masern/ (12.08.2021).
[7] Mentzer, D., Meyer, H., Keller-Stanislawski, B. Safety and tolerability of monovalent measles and combined measles, mumps, rubella and varicella vaccines. Bundesgesundheitsbl. 2013;56. Available at: https://www.pei.de/SharedDocs/Downloads/wiss-publikationen-volltext/bundesgesundheitsblatt/2013/2013-sicherheit-impfstoffe-masern-mumps-roeteln.pdf (11.08.2021).
