This fact box will help you to weigh the benefits and side effects of flu vaccination for older adults. The information and figures do not represent a final assessment. They are based on the best current scientific evidence.
The fact box was developed by the Harding Centre for Risk Literacy.
The flu is a disease caused by the influenza virus. Symptoms of influenza typically include high fever (between 38°C and 40°C or higher), headache, aching muscles, and backache, as well as cough, cold, sore throat, and hoarseness [1, 2, 3].
People with flu-like symptoms are often referred to as having the flu, without being tested for the influenza virus. Flu-like illnesses primarily include influenza and other common respiratory illnesses whose symptoms (e.g. high fever, cough) are so similar to those of influenza that the illness can only be distinguished from influenza by laboratory tests of a saliva swab or blood [3].
Influenza-like illnesses such as colds cannot be prevented by the flu vaccination.
Influenza viruses are mainly transmitted by droplets containing the virus, which are expelled especially when sneezing or coughing and can enter the respiratory tract of other people over a short distance. Contact with surfaces (e.g. door handles) or hands contaminated with secretions (saliva, sputum) that contain the virus can also cause infection [2].
For the flu vaccination, inactive or weakened influenza viruses are injected into the muscle or under the skin. The immune system then forms antibodies for this particular virus. If a person subsequently comes into contact with an active influenza virus of the same type, the immune system can react more quickly to the virus and fight it [4].
The Standing Committee on Vaccination (STIKO) of the Robert Koch Institute (RKI) particularly recommends that people with an increased risk of a severe course of the disease (e.g. older people and those with chronic illness) be vaccinated annually for seasonal influenza [2].
In past years, the annual wave of influenza has usually started after the turn of the year. Since it takes about 10 to 14 days after vaccination for the body to build up sufficient antibodies, the STIKO recommends getting vaccinated in October or November. However, it can still be useful to have the vaccination at a later time (e.g. during the flu wave) [2].
Adherence to hygiene measures, e.g. regular hand washing (especially before and after contact with people at risk) and disinfection of surfaces and objects that are likely to be contaminated (e.g. door handles) can protect against infection. Keeping away from people with flu-like symptoms and taking measures to strengthen the body's own defences (e.g. balanced diet, exercise) can also reduce the risk of infection [1].
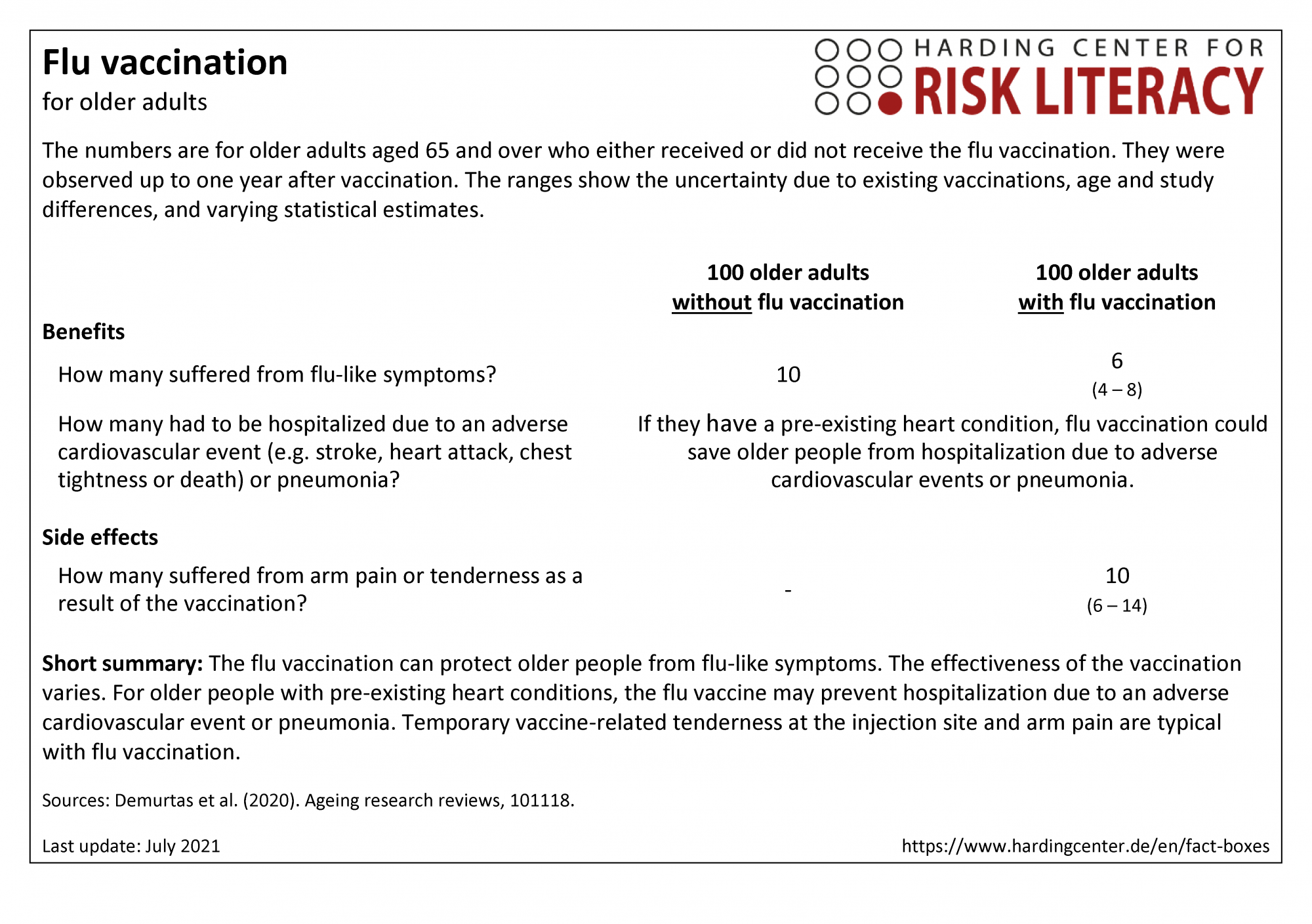
The fact box compares flu vaccination and no vaccination in terms of their benefits and side effects in older people aged 65 and over.
The table may be read as follows:
Out of every 100 healthy older adults without vaccination, about 10 suffered from flu-like symptoms within one year. In contrast, 6 out of every 100 older adults with flu vaccination suffered from flu-like symptoms [1]. This means that 4 out of every 100 older people were protected from flu-like symptoms by the flu vaccination.
The numbers in the fact box are rounded. The figures on benefits come from randomized controlled trials with a total of about 9,300 participants. The figures on side effects come from randomized controlled trials with a total of about 2,560 participants.
| Alter unter 65 Jahren | Alter ab 65 Jahren | Risikogruppen | ||
| Frauen | - | X | - | |
| Männer | - | X | - |
Erklärung der Symbole: X = für diese Personen gelten die Zahlen in der Faktenbox; (X) = auf diese Personen lassen sich die Zahlen unter Vorbehalt anwenden (in solchen Fällen ist eine Rücksprache mit ärztlichem Personal empfehlenswert); - = für diese Personen gelten die Zahlen nicht; ? = es ist unbekannt, ob die Zahlen für diese Personen gelten
The benefit of influenza vaccination depends directly on which influenza viruses are prevalent, where and how prevalent they are, and whether the vaccine used in a given flu season matches these viruses. Because the most prevalent viruses differ each year, it is impossible to accurately predict the benefit of vaccination in preventing the influenza virus for that year or when a new virus emerges [6].
Even years where the influenza vaccine is less effective, many (severe) cases of illness can nevertheless be prevented due to the frequency of influenza. In Germany, even with the current modest vaccination rates (on average about 35% of people over 65), it is estimated that about 400,000 cases of influenza are prevented each year in people over 60 [7, 8].
Infection with the influenza virus can aggravate various pre-existing conditions, especially certain heart diseases. The relationship is not yet well understood. Nevertheless, various studies indicate that influenza vaccination has a protective effect on adverse sudden cardiovascular events (e.g. stroke, heart attack, chest tightness, or death) [1].
The state of evidence was determined by the authors of the included review. According to their assessment, the overall quality of the evidence is low to moderate. It is likely that the results on influenza-like symptoms and vaccine-related adverse events will be modified by further research (moderate evidence). It is very likely that the results on protection from adverse cardiovascular events in older people with acute coronary syndrome or chronic heart disease as a result of severe influenza will be modified by further research (low-quality evidence).
- July 2021 (new search, update of evidence, update of accompanying text)
- July 2019 (update of the evidence, update of the accompanying text)
- August 2016 (development)
Information for the fact box was obtained from the following sources:
[1] Demurtas, J., Celotto, S., Beaudart, C., Sanchez-Rodriguez, D., Balci, C., Soysal, P., ... & Maggi, S. (2020). The efficacy and safety of influenza vaccination in older people: An umbrella review of evidence from meta-analyses of both observational and randomized controlled studies. Ageing research reviews, 101118. doi: 10.1016/j.arr.2020.101118.
[2] Robert Koch Institute (RKI) (2020a). Current data and information on infectious diseases and public health. Vaccination status of children and adolescents in Germany. STIKO: Influenza vaccinations in the COVID-19 pandemic. Epid Bull 32/33. https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2020/Ausgaben/32-33_20.pdf (08.06.2021).
[3] Institute for Quality and Efficiency in Health Care (IQWiG). (2019a, October 23). Flu. gesundheitsinformation.de. https://www.gesundheitsinformation.de/grippe.html (08.06.2021).
[4] Buda, S., Prahm, K., Dürrwald, R., Biere, B., Schilling, J., Buchholz, U., & Haas, W. (2018). Report on the epidemiology of influenza in Germany season 2017/18. doi: 10.17886/rkipubl-2018-003.
[5] Robert Koch Institute (RKI). (2019, 30 January). Influenza - Frequently asked questions and answers about influenza. rki.de. https://www.rki.de/SharedDocs/FAQ/Influenza/FAQ_Liste.html;jsessionid=D138845A71D1096528DD8AD29856C00E.internet052?nn=2370434 (10.06.2021).
[6] Institute for Quality and Efficiency in Health Care (IQWiG). (2019b, 23 October). How much protection does a flu vaccination offer? gesundheitsinformation.de. https://www.gesundheitsinformation.de/wie-viel-schutz-bietet-eine-grippeimpfung.html (08.06.2021).
[7] Robert Koch Institute (RKI) (2020b). Kurz & Knapp: Factsheets on vaccination. Influenza vaccination. https://www.rki.de/DE/Content/Infekt/Impfen/Materialien/Faktenblaetter/Influenza.pdf? (08.06.2021).
[8] Federal Statistical Office (Destatis) (2020). Influenza: vaccination rates of people aged 65 and over 2018, in international comparison. https://www.destatis.de/DE/Themen/Laender-Regionen/Internationales/Thema/bevoelkerung-arbeit-soziales/gesundheit/Influenza-2.html (08.06.2021).
[9] Buda, S., Dürrwald, R., Biere, B., Buchholz, U., Tolksdorf, K., Schilling, J., Goerlitz, L., Streib, V., Preuß, U., Prahm, K., Haas, W. & AGI Study Group (2021). Influenza weekly report week 20/2021; Arbeitsgemeinschaft Influenza - Robert Koch-Institut. https://edoc.rki.de/bitstream/handle/176904/8276/Influenza_Wochenbericht_KW20_2021.pdf (08.06.2021).
Documentation on how the numbers in the fact box were determined is available on request.
