This fact box will help you to weigh the benefits and side effects of a combined booster vaccination against whooping cough in adolescence and adulthood. The information and figures do not represent a final assessment. They are based on the best current scientific evidence available.
The fact box was prepared by the Harding Center for Risk Literacy.
Whooping cough (pertussis) is a highly contagious infectious disease of the upper respiratory tract caused by the bacterium Bordetella pertussis. Unlike other diseases for which there are vaccines, whooping cough cannot be eradicated.
Adolescents and adults usually experience flu-like symptoms such as a cold, weakness, and sometimes a fever, along with a cough that lasts for several weeks, with especially strong coughing attacks at night. Transmission to other people occurs through droplets that spread when sneezing, talking, or coughing. The time from infection to the appearance of the first symptoms can take one to three weeks. Even vaccinated people who do not fall ill themselves can unwittingly become carriers for a short time after contact with others who are infected. In addition, the vaccination protection is only of limited duration, so that an infection is possible despite vaccination, as is reinfection.
Babies and small children are particularly at risk from whooping cough; for them infections can be life-threatening. Very rare complications, especially in babies up to six months of age, are pneumonia, seizures, brain damage due to lack of oxygen, and, in extreme cases, death by suffocation [1].
Whooping cough vaccine is given four times in infancy and toddlerhood as part of a six-fold combination vaccination also including tetanus and diphtheria, among others. However, these and some other vaccinations provide only temporary immunity. A first booster vaccination against whooping cough is given at the age of 5 to 6 years, followed by another at the age of 9 to 16 years. For adults, the booster vaccination is recommended every 10 years. A booster vaccination "reminds" the immune system of the antigen and speeds up its response when it comes into contact with the virus.
A vaccine for whooping cough alone is no longer available in Germany. If necessary, a polio (poliomyelitis) vaccine can be given at the same time [1,2].
The Standing Committee on Vaccination (STIKO) recommends a booster vaccination against whooping cough every ten years for people aged 18 years and older. Adults can also be vaccinated against whooping cough when their next tetanus and diphtheria vaccination is due [1].
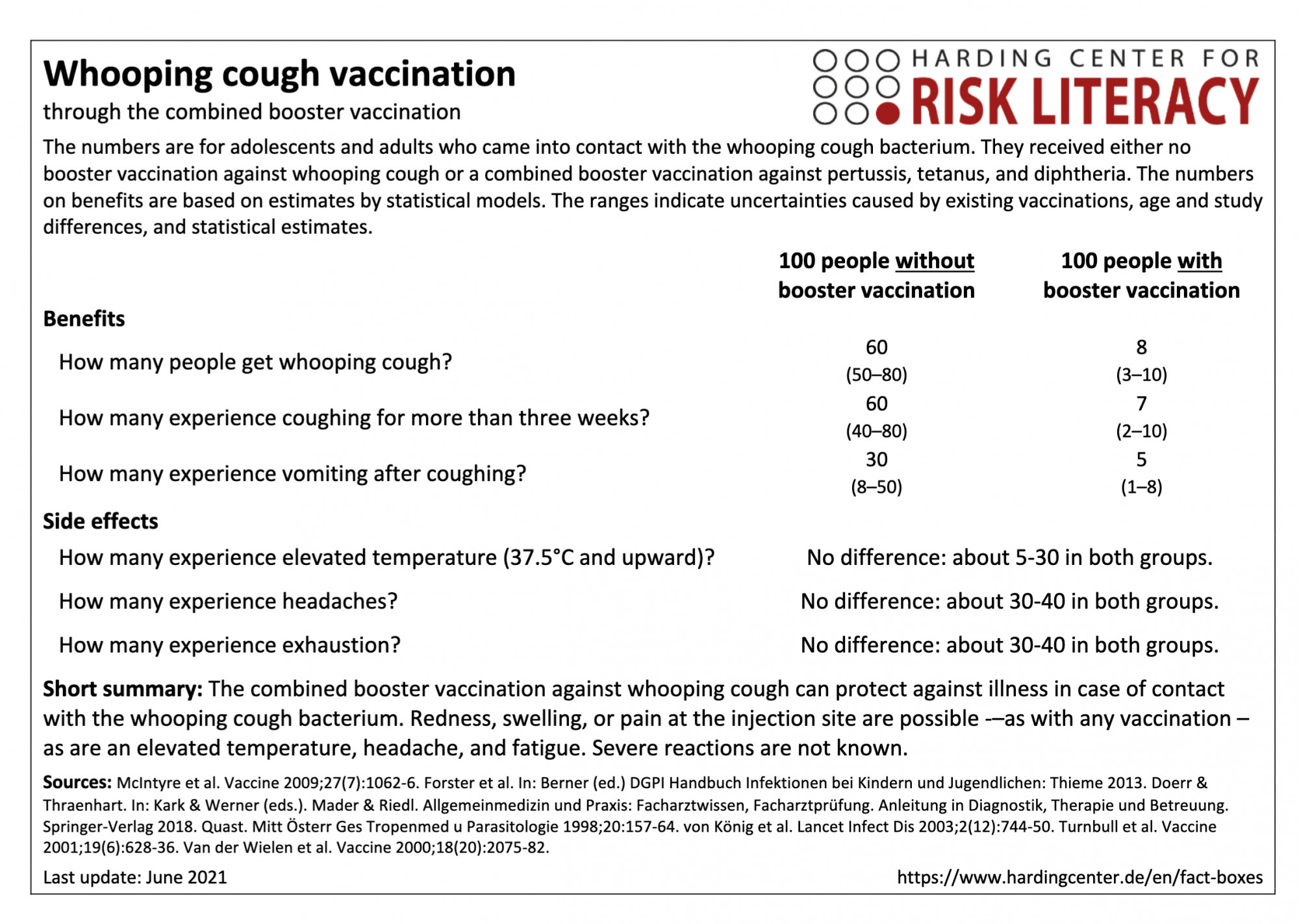
The fact box compares the benefits of combined vaccination and of non-vaccination against whooping cough as a model for adolescents and adults who come into contact with the whooping cough bacterium. In addition, the side effects of the tetanus and diphtheria vaccination are compared with those of the combined booster tetanus, diphtheria, and whooping cough vaccination. The figures on benefits are based on estimates by statistical models. The ranges show the uncertainty due to existing vaccinations, age and study differences, and statistical estimates.
The table may be read as follows:
If 100 adolescents and adults without a whooping cough booster vaccination came into contact with the whooping cough bacterium, an average of 60 (50 to 80) would likely fall ill. In contrast, an average of 8 (3 to 10) out of every 100 of those vaccinated with the booster vaccination would be infected after bacterial contact.
There are no relevant differences in the side effects of the combination vaccination against tetanus and diphtheria and the side effects of the triple vaccination against tetanus, diphtheria, and whooping cough. About 5 to 30 out of every 100 adults with tetanus and diphtheria vaccination or tetanus, diphtheria, and whooping cough vaccination had an increased body temperature.
The numbers in the fact box, which were calculated on the basis of model estimates, were rounded to the nearest tens. Figures below 10 were not rounded.
The figures on the frequency of infection after contact with the whooping cough bacterium are based on model calculations of collected data from clinical practice. This includes physician information and textbook data on contact indices (proportion of infected people among those who have contact with the bacterium) and manifestation indices (proportion of symptomatically ill people among those infected), for which no actual patient studies are available. The figures based on clinical practice do not necessarily correspond to the health status of today's population and all vaccines currently offered in Germany [3-8].
The data on side effects are based on two randomized controlled trials with a total of 1,000 participants [9, 10].
The probability of bacterial contact depends on many factors. These include, for example, the number of vaccinated people in the population and the possibilities for the bacterium to spread. Contact with the pathogen becomes less frequent if a majority of the population is vaccinated, which prevents the bacterium from spreading (herd immunity).
It can be assumed that immune protection lasts for at least five years after booster vaccination in adults [3]. Despite booster vaccination, infection can still occur [11]. Even a former infection whooping cough does not provide life-time protection against reinfection [1].
The evidence was assessed by the authors of the included review and the creators of the fact box. The overall quality of the evidence is moderate.
Some results could be modified by further research. Contagion and manifestation indices have not been explored with randomized controlled trials, but vaccine effectiveness has been confirmed across different study populations with different study designs. These at least provide evidence of a reduction in disease symptoms in vaccinated versus non-vaccinated individuals. The data on side effects are based on randomized controlled trials. It is very unlikely that further research will change the results.
- June 2021 (update of the search, no new evidence; update of the accompanying text)
- November 2020 (update of the search, no new evidence; update of the accompanying text)
- April 2016 (development)
Information for the fact boxes were obtained from the following sources:
[1] Robert Koch Institute. (2020, 6 April). RKI guidebook - Whooping cough (pertussis). rki.de. Available at: https://www.rki.de/DE/Content/Infekt/EpidBull/Merkblaetter/Ratgeber_Pertussis.html (25.05.2021).
[2] Paul Ehrlich Institute. (2021, 7 May). Diphtheria vaccines. pei.de. Available at: https://www.pei.de/DE/arzneimittel/impfstoffe/diphtherie/diphtherie-node.html (25.05.2021).
[3] McIntyre, P. B., Burgess, M. A., Egan, A., Schuerman, L., & Hoet, B. (2009). Adult booster vaccination with reduced antigen diphtheria, tetanus and pertussis vaccine: immunogenicity 5 years after vaccination. Vaccine, 27(7), 1062-1066.
[4] Forster, J., Bialek, R., & Borte, M. (2013). DGPI Handbook: Infections in children and adolescents. Georg Thieme Verlag.
[5] Doerr, H. W., & Thraenhart, O. (1988). Vaccinations and vaccination problems in the elderly. In Cancer in old age. On oncology and immunology in older age. (S. 143-147). Steinkopff.
[6] Mader, F. H., & Riedl, B. (2018). General medicine and practice: specialist knowledge, specialist examination. Guidance in diagnostics, therapy and care. Springer-Verlag.
[7] Quast, U. (1998). Side effects after vaccination an overview. Tropenmed. parasitol, 20, 157-164.
[8] Von König, C. W., Halperin, S., Riffelmann, M., & Guiso, N. (2002). Pertussis in adults and infants. The Lancet Infectious Diseases, 2(12), 744-750.
[9] Turnbull, F. M., Heath, T. C., Jalaludin, B. B., Burgess, M. A., & Ramalho, A. C. (2000). A randomized trial of two acellular pertussis vaccines (dTpa and pa) and a licensed diphtheria-tetanus vaccine (Td) in adults. Vaccine, 19(6), 628-636.
[10] Van der Wielen, M., Van Damme, P., Joossens, E., Francois, G., Meurice, F., & Ramalho, A. (2000). A randomized controlled trial of diphtheria, tetanus and acellular pertussis (dTpa) vaccine in adults. Vaccine, 18(20), 2075-2082.
[11] Ward, J. I., Cherry, J. D., Chang, S. J., Partridge, S., Keitel, W., Edwards, K., ... & APERT Study Group. (2006). Bordetella pertussis infections in vaccinated and unvaccinated adolescents and adults evaluated in a national prospective randomized Acellular Pertussis Vaccine Trial (APERT). Clinical Infectious Diseases, 43(2), 151-157.
